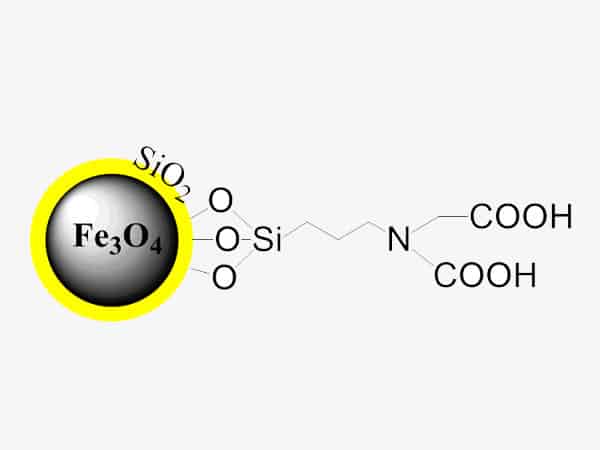
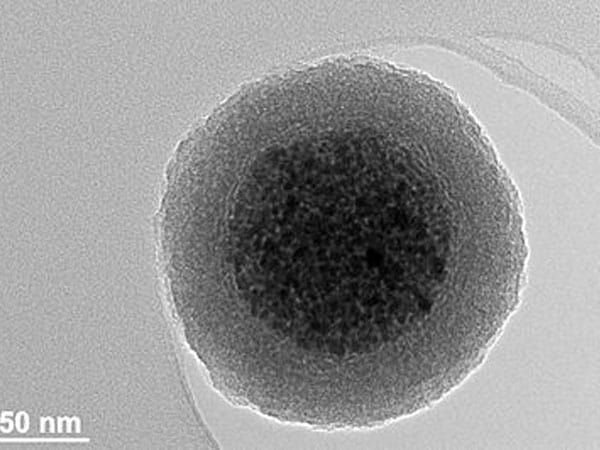
Overview of Carboxyl Magnetic Beads
The carboxyl magnetic beads use superparamagnetic Fe3O4 as the core, polystyrene coated on the outer layer, and surface modified with carboxyl groups. They have the characteristics of superparamagnetism, fast magnetic response, rich carboxyl functional groups, monodispersity and sub-micron particle size. Carboxyl magnetic beads can covalently couple peptides, proteins, oligonucleotides and other biological ligands to the surface of magnetic beads under the action of special chemical reagents (such as EDC), which is important in medical and molecular biology research vector tool.
Note: Operations such as freezing, drying and centrifugation will cause agglomeration of the carboxyl magnetic beads of the immunomagnetic beads, which is not easy to resuspend and disperse, and may affect the chemical activity of the functional groups on the surface of the magnetic beads. Before using this product, be sure to vortex sufficiently to keep the magnetic beads evenly suspended.